Introduction:
Disease-2019 (COVID-19) pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).After the adjustments and optimizations of Chinese control strategies and policies for COVID-19 on December of 2022, clinical exposure increased rapidly.There are greater concerns for patients with hematological malignancies, particularly those who have undergone hematopoietic stem cell transplantation (HSCT).
One promising preventive measure that has recently gained attention is ursodeoxycholic acid (UDCA). This drug is mainly used to treat biliary tract disease and other hepatobiliary disorders, particularly primary biliary cholangitis (Hirschfield GM, et al. Gut 2018); and also has been widely used at a higher dosage to prevent sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) after HSCT in the clinical setting. Recently, Brevini et al. demonstrated that UDCA downregulated ACE2 expression by reducing farnesoid X receptor signaling, resulting in reduced SARS-Cov-2 infection (Brevini T, et al. Nature 2022), indicating that pharmacological prophylaxis using UDCA against COVID-19 in vulnerable groups was feasible, but the clinical data to support this practice is limited.
Therefore, we aim to present epidemiological data for Chinese patients with hematological malignancies during this epidemic wave, and investigate the protection effects offered by UDCA against COVID-19 in these patients.
Methods:
This investigation was a single-center, observational real-world study, and was granted by the ethics committee of the Institute of Hematology & Blood Diseases [No. QTJ2023012-EC-1]. The transplant patients were included in the “NICHE-SKIRT” project (Cao Y, et al. Am J Hematol 2023). Informed consent for the acquisition and the use of patient samples was obtained.
Data for hospitalized patients at the Institute of Hematology & Blood Diseases Hospital (Tianjin, China) was reviewed between December 2022 and January 2023. COVID 19 status was assessed using hospital-reported PCR and/or antigen tests for twice a week. We analyzed follow-up records up to 14th February 2023. The primary end-point was the rate of COVID -19 infection.
Results:
We enrolled 393 patients with a median age of 38 years old. At the cutoff date, 297 patients (75.6%) had confirmed COVID-19 infection (72.5% in the allo-HSCT cohort). Among the 273 evaluable patients, the majority (64.8%) had asymptomatic/mild disease. The severe/critical patients accounted for only 15.8%. Seven hospitalized patients had died from COVID-19 by the last follow-up date. The median period between COVID-19 diagnosis and death was 15 days (range: 2-32 days).
Lower proportion of CD3+CD4+T cells associated with more severe COVID-19 clinical spectrum (Asymptomatic/mild [n = 97] moderate [n = 46], adjusted P = 0.001; Asymptomatic/mild vs. severe/critical [n = 25], adjusted P = 0.036).
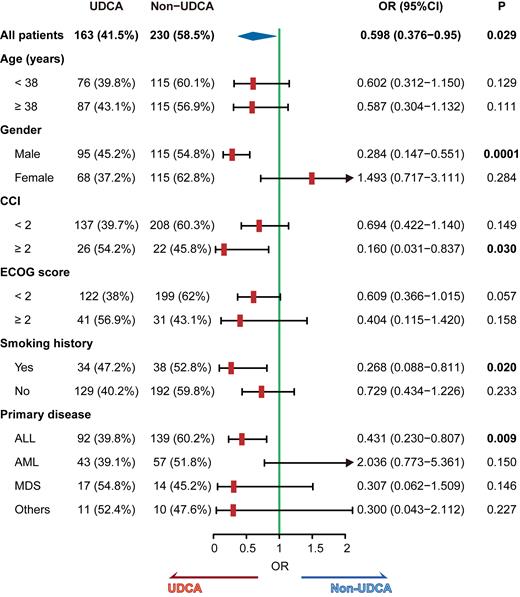
Among these patients, 163 received UDCA (UDCA group) while 230 did not (non-UDCA group). The COVID-19 infection rate was lower in the UDCA group (69.9% vs. 79.6%; P= 0.039). However, patients in the UDCA group had a higher ECOG score and Charlson Comorbidity Index (Table 1), suggesting that they were more likely to have underlying health conditions. To account for this potential bias, we performed PSM, which confirmed that the UDCA group had a significantly lower rate of COVID-19 infection compared to the non-UDCA group (69.0% vs. 81.0%; P = 0.049) in the validated cohort.
Univariate logistic regression analysis showed that only UDCA administration was associated with COVID-19 infection (OR: 0.598; P = 0.029). To further investigate the effect of UDCA in specific populations, we carried out an exploratory analysis. Our results suggest that males, patients with a CCI ≥ 2, a history of smoking, and those with acute lymphoblastic leukemia (ALL) may benefit the most from the protective effect of UDCA against COVID-19.
Conclusion:
This study aimed to investigate the prevalence and clinical outcomes of SARS-CoV-2 infections in Chinese patients with hematologic disorders during the COVID-19 epidemic that began at the end of 2022. Our findings provide support for preclinical studies suggesting that UDCA may have marginal benefit in protecting against COVID-19. However, prospective studies with a higher dose of UDCA need to be performed.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Ursodeoxycholic; for SOS/VOD prophylaxis following HSCT in the clinical setting

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal